- Research
- Open access
- Published:
Hygiene-based measures for the prevention of cytomegalovirus infection in pregnant women: a systematic review
BMC Pregnancy and Childbirth volume 24, Article number: 172 (2024)
Abstract
Background
Human Cytomegalovirus (HCMV) is the most frequent congenital infection worldwide causing important sequelae. However, no vaccine or antiviral treatments are currently available, thus interventions are restricted to behavioral measures. The aim of this systematic review was to assess evidence from available intervention studies using hygiene-based measures to prevent HCMV infection during pregnancy.
Methods
Studies published from 1972 to 2023 were searched in Medline, PsycInfo, and Clinical Trials (PROSPERO, CRD42022344840) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Methodological quality was assessed by two authors, using ROBE-2 and MINORS.
Results
After reviewing 6 selected articles, the outcome analysis suggested that implementation of hygiene-based interventions during pregnancy prevent, to some extent, the acquisition of congenital HCMV.
Conclusions
However, these conclusions are based on limited and low-quality evidence available from few studies using this type of intervention in clinical practice. Thus, it would be necessary to perform effective and homogeneous intervention studies using hygiene-based measures, evaluated in high-quality randomized controlled trials (RCTs).
Background
Human Cytomegalovirus (HCMV) belongs to the Herpesviridae family (HHV-5), and it is the most frequent congenital infection worldwide (0.5 to 5%), causing important sequelae such as sensorineural hearing loss (SNHL) and neurodevelopmental disabilities in newborns [1,2,3]. In reproductive age women, HCMV seroprevalence varies based on socioeconomic factors [4]. While in developed countries in Europe and North America, seroprevalence ranges from 40 to 83% [5, 6], in developing countries from Asia, Africa, and Latin America, seroprevalence can reach 100% [7]. The prevalence of congenital infection (cHCMV) is therefore associated with seroprevalence among pregnant women, with a higher seroprevalence correlating with ahigher prevalence of cHCMV infection [8]. In developed countries where up to 50% of childbearing age women are seronegative, cHCMV infection usually occurs due to frequent contact with small children (< 3 years of age) [9, 10].
During primary infection, viral shedding can occur through saliva, urine, breast milk, semen, and blood [11]. A primary infection takes place when an individual with no previous HCMV infection (HCMV seronegative individuals) are infected through contact with a HCMV infected individual, triggering a broad immune response and establishing lifelong latency [12]. Seropositive individuals can develop new HCMV infection episodes from viral reactivation of latent infection or through re-infection with different viral strains [12,13,14]. During pregnancy, HCMV transmission to the fetus can occur both from mothers with primary infection (seronegative women) and from mothers with reactivated latent infection where hormonal changes associated with pregnancy and lactation may stimulate the reactivation [15, 16].
The greatest risk of vertical transmission is associated with primary infection ranging from 30 to 35%, compared to 1.1 to 1.7% for non-primary infections [17]. However, it is crucial to consider the likelihood of acquiring an infection. The risk appears to be relatively low for seronegative women and relatively high for seropositive women [18]. This observation is supported by earlier studies that modelled the force of infection, estimating a higher incidence in highly seropositive populations [19, 20], likely due to environmental and behavioural differences. Some studies indicate that the risk of re-infection among seropositive women surpasses the combined risks of both acquisition and maternal-to-fetal transmission among seronegative women [21]. Recent serological studies examining strain-specific HCMV antibody responses have revealed that maternal re-infection with a new strain is a significant factor in congenital infection among seropositive women, with re-infections occurring in 8% of seroimmune pregnancies [22].
Other authors have reported a much greater proportion of symptomatic cHCMV linked to reinfection during pregnancy [23, 24]. Up to 10% of neonates with cHCMV infection are symptomatic and develop different sequelae. cHCMV is the leading cause of sensorineural hearing loss (SNHL) and neurodevelopmental delay, with a large number of symptomatic children presenting some degree of psychomotor and cognitive disabilities, and with visual impairment in up to 50% of infants [25,26,27,28]. Likewise, several studies have demonstrated that the risk of long-term neurodevelopmental sequelae, specifically hearing loss, is comparable in infants born to women with primary HCMV and those with non-primary HCMV infections during pregnancy [29,30,31]. The burden of disease related to cHCMV infection is notable, and as a consequence, infants often require special care related to therapeutic and educational needs [3, 32].
Currently, there is no licensed vaccine to prevent HCMV infection and no approved treatments to prevent viral transmission from mother to fetus [19, 33,34,35,36,37,38]. Neonates with symptomatic infection can be treated with ganciclovir and/or valganciclovir for 6 months [28]. Although this therapy has been shown to modestly reduce the incidence of hearing loss [39], follow-up duration is limited [40] and further research may be needed. Furthermore, paediatric administration of ganciclovir or valganciclovir is associated with neutropenia, and monitorization of neutrophil counts is recommended in treated children [39, 41]. Thus, to reduce transmission from the mother to the fetus and consequently reduce the global burden of HCMV disease in this population, current interventions are restricted to behavioural changes to promote prevention measures. The literature has highlighted three types of prevention measures: universal (targeting the general population, not directed to a specific risk group), selective (targeting individuals at higher-than-average risk for HCMV infection) and indicated (individuals who are identified as having an increased vulnerability for HCMV infection). In our case, the universal group would be pregnant women; the selective group would be seropositive pregnant women for HCMV infection, and the indicated group those pregnant seronegative to HCMV and, thus they are at higher risk of transmission. Selective and indicated prevention strategies might involve more intensive interventions. To identify effective intervention studies, the studies should have described which types of hygiene prevention measures are adequate, determined the preventive effect of the interventions to avoid infection, and generated high level evidence.
To the best of our knowledge, only two review manuscripts describing HCMV preventive interventions have been conducted to date in the general population [42, 43]. However, neither of the systematic reviews established which type of intervention is most appropriate for preventing HCMV infection. The first review focused exclusively on trials published before 2004 [42] while the second focused on trials published before 2019 [43]. Although both studies included content on behavior modifications, none of them used the Psyinfo database specialized in this field.
Thus, the existing evidence on preventive interventions for HCMV infection in the perinatal period remain inconclusive. For this reason, the aim of this review is to collate evidence relating hygienic measurements acquisition and counselling during pregnancy in order to reduce cHCMV infection.
Methods
Search procedures and eligibly criteria
This systematic review of published studies was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement [20, 44]. A systematic review between 1972 and 2023, written in English (due to resource limits), assessing preventive intervention for HCMV infection was carried out. Database search was conducted in September 2023 by two authors (MFR and EGR) independently.
A protocol was elaborated to implement different steps underlying this systematic review and was registered on PROSPERO, the International Prospective Register of Systematic Reviews (ID CRD42022344840). No deviations from the protocol have occurred.
A total of three electronic databases were searched: MEDLINE, PsycINFO. In addition, CLINICAL TRIALS.gov was used to identify unpublished studies or studies still ongoing. The following search terms were combined: (“cytomegalovirus” OR “CMV” OR “CCMV” OR “HCMV” OR “Human betaherpesvirus 5” OR “cytomegalovirus infection*” OR “CMV infection*” OR “cCMV infection*” OR “HCMV infection*” OR “Human betaherpesvirus 5” infection*” OR “congenital cytomegalovirus infection*” OR “congenital infection*” OR “congenital CMV” OR “congenital HCMV” ) AND (“prenatal*” OR “pre-natal*” OR “pre natal*” OR “antenatal* OR “ante-natal*” OR “antepartum” OR “ante-partum” OR “pregnancy” OR “pregnant*” OR “mother*” OR “childbearing” OR “woman” OR “women”) AND (“prenatal education*” OR “antenatal education*” OR “birth preparation*” OR “prenatal class*” OR “antenatal class*” OR “health education*” OR “health promotion*” OR “counselling*” OR “hygiene*” OR “hand wash*” OR “wash hand*” OR “program” OR prevention” OR “control” OR “hygiene-based” OR “control” OR “hygiene education” OR “behavioral intervention” OR “Vertical prevention” ).
Inclusion and exclusion criteria
Analysis of the articles was performed based on previously established inclusion and exclusion criteria and the availability of the full text in English. Randomized controlled trial (RCT) and non-RCT about the effectiveness of HCMV acquisition were eligible for inclusion in the systematic review. The review included articles studying adult pregnant women or attempting pregnancy to whom preventive intervention based on hygiene education and control groups receiving standard treatment or information. The primary outcome of the review was the measurement of seroconversion rates and the secondary was the adherence of pregnant women to the intervention.
Search results were exported to an Excel file and duplicate manuscripts were removed. Two authors (MFR and EGR) independently screened titles and abstracts for eligibility and the full text of the potentially eligible articles were screened. Studies were excluded if they did not evaluate the effectiveness of preventive intervention for HCMV, or did not include psychological or biological outcomes. Any disagreement was resolved by discussion.
Data extraction
Two reviewers (MFR and EGR) independently extracted data from each included study and checked for accuracy using a data extraction excel spreadsheet. The following data were extracted: aim of study, author, year of publication, country of study, time of study, study design, inclusion/exclusion criteria, characteristics of cohort, description of interventions’ characteristics (data were collected as narrative results) – type (information or counselling); delivery format (face-to-face, written, video, individual, group, online); time of intervention (pregnancy or attempting pregnancy); duration of intervention (range); number of sessions (range); follow-up duration; providers; outcome measures and main findings. Summary tables were made to create the extracted information in an organized presentation. Excluded studies and reason for the exclusion has been included in Supplementary material (Table S1).
Risk of bias assessment
Methodological quality of the included studies was independently assessed by two reviewers (MFR and EGR) using ROB-2 [45], a tool developed for assessing the quality of randomized health care interventions and MINOR [46] for non/randomized intervention [47]. ROB-2 evaluates five domains of research validity and bias: randomization process, deviations from intended interventions, missing outcome data, outcome measurement and selection of the reported results. Studies were evaluated as either low, some concerns or high risk of bias for each domain. MINORS contained 12 items, the first eight being specifically for non-comparative studies. The items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The global ideal score being 16 for non-comparative studies and 24 for comparative studies.
Risk of bias was categorized as low or high. Disagreements on quality rating were discussed and a consensus was reached.
Results
Identification of studies
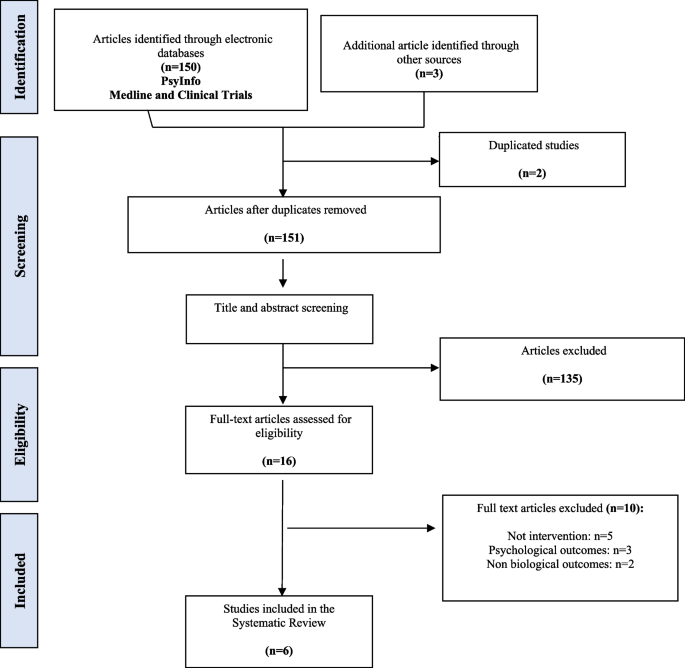
Search results are summarized in the PRISMA flowchart (Fig. 1). The initial search identified a total of 150 references and 3 additional records were collected based on experts in the field. After removing duplicate references, the title and abstract, a total of 145 references were screened (first screening) and a total 135 studies were excluded. A full-text review was performed for the remaining 16 references (second screening) and based on eligibility assessment 10 records were excluded (exclusion reasons are presented in Table S1) and six articles were included in the systematic review. The methodological quality and bias risk of the included studies are shown in Table 1. All four non-RCT papers [48,49,50,51] were classified as critically low-quality using MINORS, mainly due to unbiased assessment of the study, for lacking a follow-up period or a prospective calculation of the sample size. In contrast, the two RCT papers [52, 53] were evaluated with a high quality based on ROBE-2 criteria.
Study characteristics
Characteristics of the six included papers are shown in Table 2. All the studies were published between 2004 and 2021 reporting the findings from a total number of 10,197 participants (i.e., pregnant women or women who attempt to be pregnant). The studies were carried out in United States [52]; Italy [49]; Israel [48]; United Kingdom [53] and France [50, 51]. Sample size ranged from 103 to 5173 women with a median of 545 and a mean of 1699.5.
As previously stated, two of the studies were RCTs [52, 53] while the remaining four paper were a retrospective cohort study [48]; an observational controlled study [49] and two prospective cohort studies [50, 51]. Four studies focused exclusively on pregnant women [49,50,51, 53]. One study on women who were planning to be pregnant [48] and the last one included both, pregnant women and women who were planning to be pregnant [52].
Regarding the type of prevention approach used, five studies included selective prevention in seronegative women [49,50,51,52,53] and finally one paper focused on universal prevention [48].
Regarding the type of personnel providing the interventions, in four of the studies the interventions were mainly provided by health professionals (nurses, midwives, gynecologists) while in two of the studies this information was not reported [49, 53].
Characteristics of the interventions
All six studies evaluated the effectiveness of the preventive intervention on the reduction of HCMV acquisition (seroconversion). Most of the papers focused on informing patients of preventive measures [49,50,51,52,53]. However, some of the studies in addition to informing patients of preventive measures, included other parameters such as adherence to follow up visits [52], patient follow up by telephone calls [51] and patient reinforcement [49]. Only one of the selected studies included psychological support through counseling [48].
Table 3 shows a summary of the main results. In more detail, Adler et al., (2004) analyzed 166 seronegative women with a child below 36 months of age. In this work, participants were randomly assigned to either the control group (intervention) or the intervention group (full intervention). In the control group, women received basic information about HCMV infection but they were not aware of their serological status or whether the child was shedding HCMV or not. On the contrary, women assigned to the intervention group received the same information and indications as in the control group but additionally they were aware of their serological status and whether their child was shedding HCMV or not, and their implications. In addition, home visits were carried out every 3 months in order to assess adherence to the measures of both groups.
Calvert et al., 2021 enrolled 103 pregnant women living with children less than four years old that were randomly divided in the control and the intervention groups. The control group received information through a series of slides about influenza vaccination during pregnancy while intervention group watched a digital educational film with detailed information about HCMV infection and its prevention.
Picone et al., (2009) recruited 3665 seronegative pregnant women during the first trimester visit to the obstetrician. Detailed oral and written information about HCMV infection and its prevention were given to both parents and at around 36 weeks of gestation, a second HCMV serologic test was performed. Following the same procedure for the intervention, Vauloup-Fellous et al., (2009) enrolled 5173 seronegative pregnant women during their first trimester visit to the obstetrician.
Reichman et al., (2014) carried out a retrospective cohort study of 444 women who were attempting pregnancy and were referred to a fertility clinic. Seventy-two seronegative women received detailed preconception counselling about HCMV infection and its preventive measures and every 3–4 months they had a follow-up HCMV serology test.
Finally, Revello et al., (2015) included 646 pregnant women with a control group integrated by women enrolled at delivery who were not informed about HCMV infection, while the intervention group received information about hygiene measures and were prospectively tested for HCMV infection until delivery. Furthermore, in this study authors carried out a reinforcement strategy through sessions during follow-up visits at 18 weeks of gestation and questionnaires every 6 weeks.
Effectiveness of the interventions on HCMV acquisition
The six selected studies reported the HCMV-specific seroconversion rates as a function of the intervention. Of the three studies with a reported control group [49, 52, 53], only one indicated significantly lower HCMV infection after the intervention (4/331, 1.2%) compared with the control group (24/315, 7.6%) [49]. While no significant reduction of seroconversion was found in Adler et al., (2004) and Calvert et al., (2021) when comparing the control and intervention groups. Regarding the study performed by Adler et al., (2004), a significant reduction in HCMV infection was reported in pregnant women with children younger than 36 months of age who were shedding HCMV (1/17, 5.9%) compared to women with children younger than 36 months of age shedding HCMV attempting pregnancy (10/24, 41.6%). On the other hand, Calvert et al., (2021) reported no significant differences in the seroconversion rate between the end of the first trimester and 34 gestational weeks was 4.55% (2/44) in the intervention group and 4.65% (2/43) in the control group.
The study performed by Picone et al., (2009) reported a reduction in seroconversion after the intervention (5/1951, 0.26%) between 12 and 36 weeks of gestation compared with the first trimester of pregnancy (9/1960, 0.46%). Assuming the nine patients with primary infections had negative serology at 0 weeks of gestation (WG), the count of women without prior HCMV exposure at the start of pregnancy would be the sum of seronegative women at 12 WG (1951) plus nine, totaling 1960.
Similar results were obtained in the study reported by Vauloup-Fellous et al., (2009) in which a significant reduction was also observed after intervention at 12 weeks of gestation (5/2583, 0.19%) when compared with the period before the intervention (11/2594, 0.42%). If we consider that the 11 patients with primary infection (indicated by a low HCMV-G avidity index) had negative serology at 0 weeks of gestation, the number of women without prior HCMV exposure at the beginning of pregnancy would be the total of seronegative women at 12 weeks of gestation (2583) plus 11, amounting to 2594.
Finally, although no comparison was made in the study performed by Reichman et al., (2014), none of the 69 seronegative women who were followed-up until the end of the study seroconverted after receiving counselling at the preconception visits.
Adherence, changes in behavior and HCMV perception
Most of the studies did not report information related to behavioral changes of perception of HCMV [48, 50, 51, 53] as secondary outcomes. Regarding adherence, it is important to evaluate how well pregnant women follow recommended preventive measures as advised by healthcare providers. Two studies provided information regarding adherence to treatment [49, 52]. In Adler et al., (2004), authors reported no significant differences in adherence to the intervention between the groups of participants (infected and uninfected women and pregnant and attempting pregnancy women). On the other study, 745/932 (80%) of respondents women described following the recommendations often 492/745 (66%) or always 253/745 (14%) during pregnancy being the lack of time the major cause to reduce adherence to the prevention measures [49].
Discussion
Despite the great Public Health impact caused by HCMV congenital infections as a leading cause of stillbirth, neurodevelopmental problems and hearing loss worldwide, there are no vaccines or therapies commercially available to prevent the infection [19, 33,34,35]. With this regard, the implementation of hygienic measures in the population at risk stands as the cornerstone to prevent HCMV transmission from the mother to the fetus during pregnancy.
In summary, the findings from this systematic review indicate that incorporating hygiene-focused interventions during pregnancy can to some degree reduce the likelihood of acquiring congenital HCMV infection. Nevertheless, the review highlights a scarcity of studies on preventive measures, and the existing ones vary significantly in terms of target populations, assessed outcomes, and the nature and conditions of implemented interventions. This heterogeneity poses challenges in drawing conclusive insights from the available evidence.
The prevalence of cHCMV infection varies from 0.2 to 2% (average 0.65%) depending on maternal seroprevalence [54]. However, this data come mainly from studies performed in developed regions such as Europe, the USA, and Japan. In low income countries the cHCMV prevalence is higher varying from 6 to 14% [55,56,57,58,59]. The 6 studies included in this systematic review were carried out in five developed countries, and results may therefore not be applicable to other countries with higher prevalence rates. Our results highlight the urgent need to conduct new studies implementing preventive measures in the population at higher risk of infection and transmission. Furthermore, in developing countries in addition to the higher prevalence rates, promoting and implementing hygiene-based measures may be more difficult based on lower socio-economic conditions. In fact, it has been reported that higher educational and social levels are associated with improved patient possibilities to change health behaviours [60, 61].
Regarding the HCMV transmission, in three out of the six studies, seroconversion rates were significantly lower either in the intervention group [49] or after the implementation of hygiene-based measures [50, 51]. It is important to mention that, similarly to previously reported results, the three studies with a significant reduction in HCMV transmission rate were conducted exclusively in pregnant women, [43]. Pregnancy has been commonly defined as a ‘teachable moment” since women are more motivated to improve both the lifestyle and healthy habits compared with non-pregnant women [60, 62, 63]. The response based on emotions during early pregnancy leading to concerns about the fetus health together with the new social role of becoming a mother can motivate pregnant women to modify their lifestyle habits [64]. Based on pregnant women’s interest in maintaining healthy behaviours in this period, implementing protocols to improve the knowledge regarding the HCMV transmission will be ideal and will also increase available evidence of the effectiveness of the preventive intervention. More information is required regarding the following aspects: moderators and mediators of the prevention treatment response, contents, format and adherence to the preventive measures.
In addition to the significant reduction in the seroconversion rate observed after our analysis, the extrapolation of results may not be possible due to the limited number of RCTs, the small sample size and the heterogeneity of the sample. Thus, as previously stated, our results highlight the urgent need to carry out new RCTs involving pregnant women from different socio-economic backgrounds. In addition, except for one study [48], the interventions were based on informing patients of preventive measures instead of counselling and active behavior-changing interventions which have already proven to be effective promoting heathy habits during pregnancy for other pathologies [65,66,67]. Additionally, counselling is a complex process and the effectiveness of the intervention may be dependent on the specific training and experience of the provider. In the six selected studies, interventions were primarily administered by healthcare professionals such as nurses, midwives, and gynaecologists, potentially lacking specialized training for HCMV infection. Furthermore, these studies lacked clear delineation of the specific interventions, often providing imprecise descriptions, thereby complicating the drawing of definitive conclusions. Consequently, the involvement of professionals specialized in behavior modification could prove instrumental in crafting effective health prevention strategies, proposing and implementing tailored counselling plans during pregnancy.
Limitations
Some limitations must be considered to interpret our results correctly. (i) Our results may be biased since studies were carried out in high-income regions which make difficult the extrapolation of the results to developing countries. (ii) The number of available studies was small and in some studies the sample size was also reduced, leading to limited representativeness [52, 53]. (iii) It was not possible to conduct a meta-analysis due to the clinical and methodological heterogeneity of the included studies.
Conclusions
The findings presented in this review highlight the limited and low-quality published evidence currently available, limiting the possibility to make recommendations for clinical intervention. There are only six studies that met the criteria, mainly non-RCT, to study interventions aiming to prevent HCMV infection during pregnancy. It is urgent to develop effective and homogeneous interventions, evaluated in high-quality RCTs. The high number of pregnant women developing complications associated with cHCMV infection worldwide and their clinical burden highlights the need that policymakers should seek to promote research efforts in this area, for example, supporting specialized funding calls. This is particularly important due to the healthcare-related costs associated to this infection. In this sense, further efforts should be done to inform and raise awareness in society about HCMV infection during pregnancy, regardless the serostatus of women, because the associated risk cannot be minimized if they are unknown for the population at risk. Furthermore, interventions need to be replicable, based on theory and evidence, and the study of their effectiveness should be assessed in terms of time, contents and format of the intervention.
Implementation of hygienic measures in pregnant women has potential as the cornerstone to prevent HCMV transmission from the mother to the fetus during pregnancy. Nonetheless, due to the lack of evidence related to the small number and low-quality studies carried out to date, it is not possible to indicate its clinical use, and further studies are proposed with the purpose of clarifying the possible benefits.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- HCMV:
-
Human cytomegalovirus
- RCT:
-
Randomized controlled trials
- SNHL:
-
Sensorineural hearing loss
- cHCMV:
-
Congenital infection
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analysis
References
Dreher AM, Arora N, Fowler KB, Novak Z, Britt WJ, Boppana SB, et al. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr. 2014;164:855–9.
Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The Silent global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102.
Britt WJ. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol. 2017;91:e02392-02316.
Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29:e2034.
Yinon Y, Farine D, Yudin MH. Screening, diagnosis, and management of cytomegalovirus infection in pregnancy. Obstet Gynecol Surv. 2010;65:736–43.
Puhakka L, Lappalainen M, Lönnqvist T, Niemensivu R, Lindahl P, Nieminen T, et al. The burden of congenital cytomegalovirus infection: a prospective cohort study of 20 000 infants in Finland. J Pediatr Infect Dis Soc. 2019;8:205–12.
Marin LJ, Santos De Carvalho Cardoso E, Bispo Sousa SM, Debortoli De Carvalho L, Marques Filho MF, Raiol MR, et al. Prevalence and clinical aspects of CMV congenital infection in a low-income population. Virol J. 2016;13:1–5.
de Vries JJC, van Zwet EW, Dekker FW, Kroes ACM, Verkerk PH, Vossen ACTM. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol. 2013;23:241–9.
Pinninti SG, Ross SA, Shimamura M, Novak Z, Palmer AL, Ahmed A, et al. Comparison of saliva PCR assay versus rapid culture for detection of congenital cytomegalovirus infection for the national institute on deafness and other communication disorders CMV and hearing multicenter screening (CHIMES) Study. Pediatr Infect Dis J. 2015;34:536–7.
Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-chain-reaction assay for Cytomegalovirus Screening in newborns. N Engl J Med. 2011;364:2111–8.
Mocarski ES, Shenk TE, Pass RF. Cytomegaloviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Wilkins, Lippincott Williams; 2007. p. 2702–72.
Forte E, Zhang Z, Thorp EB, Hummel M. Cytomegalovirus latency and reactivation: an intricate interplay with the host Immune Response. Front Cell Infect Microbiol. 2020;10: 130.
Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J Infect Dis. 2010;202:1800–3.
Britt WJ. Maternal immunity and the natural history of congenital human cytomegalovirus infection. Viruses. 2018;10:405.
Marsico C, Kimberlin DW. Congenital cytomegalovirus infection: advances and challenges in diagnosis, prevention and treatment. Ital J Pediatr. 2017;43:1–8.
Dorfman JR, Balla SR, Pathirana J, Groome MJ, Madhi SA, Moore PL. In Utero Human Cytomegalovirus infection is Associated with increased levels of putatively protective maternal antibodies in Nonprimary infection: evidence for boosting but not Protection. Clin Infect Dis. 2021;73:E981-987.
Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005;13:164–74.
Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol. 2015;204:263–71.
Nigro G, Adler SP, La Torre R. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62.
Cajal B, Jiménez R, Gervilla E, Montaño JJ. Doing a systematic review in health sciences. Clin Y Salud. 2020;31:77–83.
Shaamash AH, Mohamed IS, Hasan MA, Ibrahim MA. Preconceptional immunity to cytomegalovirus and the risk of symptomatic congenital infection. Int J Gynecol Obstet. 2003;83:199–201.
Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, de Oliveira P, et al. F, Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol. 2010;202:297.e1-297.e8.
Mack I, Burckhardt M-A, Heininger U, Prüfer F, Schulzke S, Wellmann S. Symptomatic congenital cytomegalovirus infection in children of Seropositive Women. Front Pediatr. 2017;5: 134.
Puhakka L, Renko M, Helminen M, Peltola V, Heiskanen-Kosma T, Lappalainen M, et al. Primary versus non-primary maternal cytomegalovirus infection as a cause of symptomatic congenital infection–register-based study from Finland. Infect Dis (Auckl). 2017;49:445–53.
Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-Year prospective study of Sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153:84–8.
Xia W, Yan H, Zhang Y, Wang C, Gao W, Lv C, et al. Congenital human cytomegalovirus infection inducing Sensorineural hearing loss. Front Microbiol. 2021;12: 649690.
Pesch MH, Saunders NA, Abdelnabi S. Cytomegalovirus infection in pregnancy: Prevention, Presentation, Management and neonatal outcomes. J Midwifery Women’s Heal. 2021;66:397–402.
Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17:e177-188.
Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56:1232–9.
Ross SA, Fowler KB, Ashrith G, Stagno S, Britt WJ, Pass RF, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332–6.
Demmler-Harrison GJ, Miller JA. Maternal cytomegalovirus immune status and hearing loss outcomes in congenital cytomegalovirus-infected offspring. PLoS ONE. 2020;15: e0240172.
Chiopris G, Veronese P, Cusenza F, Procaccianti M, Perrone S, Daccò V, et al. Congenital cytomegalovirus infection: update on diagnosis and treatment. Microorganisms. 2020;8: 1516.
Griffiths P. New vaccines and antiviral drugs for cytomegalovirus. J Clin Virol. 2019;116:58–61.
Plotkin SA, Boppana SB. Vaccination against the human cytomegalovirus. Vaccine. 2018. https://doi.org/10.1016/j.vaccine.2018.02.089.
Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316–26.
De Santis M, Apicella M, De Luca C, D’Oria L, Valentini P, Sanguinetti M, et al. Valacyclovir in primary maternal CMV infection for prevention of vertical transmission: a case-series. J Clin Virol. 2020;127(March):104351.
Shahar-Nissan K, Pardo J, Peled O, Krause I, Bilavsky E, Wiznitzer A, et al. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:779–85.
Egloff C, Sibiude J, Vauloup-Fellous C, Benachi A, Bouthry E, Biquard F, et al. New data on efficacy of valacyclovir in secondary prevention of maternal–fetal transmission of cytomegalovirus. Ultrasound Obstet Gynecol. 2023;61:59–66.
Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372:933–43.
Leung J, Grosse SD, Yockey B, Lanzieri TM. Ganciclovir and Valganciclovir Use among infants with congenital cytomegalovirus: data from a Multicenter Electronic Health Record Dataset in the United States. J Pediatr Infect Dis Soc. 2022;11:379–82.
Nigro G, Scholz H, Bartmann U. Ganciclovir therapy for symptomatic congenital cytomegalovirus infection in infants: a two-regimen experience. J Pediatr. 1994;124:318–22.
Harvey J, Dennis CL. Hygiene interventions for prevention of cytomegalovirus infection among childbearing women: systematic review. J Adv Nurs. 2008;63:440–50.
Barber V, Calvert A, Vandrevala T, Star C, Khalil A, Griffiths P, et al. Prevention of Acquisition of Cytomegalovirus infection in pregnancy through Hygiene-based behavioral interventions: a systematic review and Gap Analysis. Pediatr Infect Dis J. 2020;39:949–54.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Reichman O, Miskin I, Sharoni L, Eldar-Geva T, Goldberg D, Tsafrir A, et al. Preconception screening for cytomegalovirus: an effective preventive approach. Biomed Res Int. 2014;2014:14–6.
Revello MG, Tibaldi C, Masuelli G, Frisina V, Sacchi A, Furione M, et al. Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine. 2015;2:1205–10.
Picone O, Vauloup-Fellous C, Cordier A, Parent Du Châtelet I, Senat M, Frydman R, et al. A 2‐year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG Int J Obstet Gynaecol. 2009;116:818–23.
Vauloup-Fellous C, Picone O, Cordier AG, Parent-du-Châtelet I, Senat MV, Frydman R, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? J Clin Virol. 2009;46(SUPPL. 4):S49-53.
Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J Pediatr. 2004;145:485–91.
Calvert A, Vandrevala T, Parsons R, Barber V, Book A, Book G, et al. Changing knowledge, attitudes and behaviours towards cytomegalovirus in pregnancy through film-based antenatal education: a feasibility randomised controlled trial of a digital educational intervention. BMC Pregnancy Childbirth. 2021;21:565.
Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis. 2014;22:44–8.
Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area, China. J Clin Virol. 2007;40:180–5.
Yang TH, Huang HM, Hsu WC, Tsao PN, Liu TC, Hsu CJ, et al. The prevalence and demographic features of congenital cytomegalovirus infection in an urban area of East Asia: a population-based study. PLoS ONE. 2021;16: e0248801.
Rico A, Dollard SC, Valencia D, Corchuelo S, Tong VT, Laiton-Donato K, et al. Epidemiology of cytomegalovirus infection among mothers and infants in Colombia. J Med Virol. 2021;93:6393–7.
Pathirana J, Groome M, Dorfman J, Kwatra G, Boppana S, Cutland C, et al. Prevalence of congenital cytomegalovirus infection and Associated Risk of in Utero Human Immunodeficiency Virus (HIV) Acquisition in a High-HIV prevalence setting, South Africa. Clin Infect Dis. 2019;69:1789–96.
Mwaanza N, Chilukutu L, Tembo J, Kabwe M, Musonda K, Kapasa M, et al. High rates of congenital cytomegalovirus infection linked with maternal HIV infection among neonatal admissions at a large referral center in sub-saharan Africa. Clin Infect Dis. 2014;58:728–35.
Lindqvist M, Lindkvist M, Eurenius E, Persson M, Mogren I. Change of lifestyle habits – motivation and ability reported by pregnant women in northern Sweden. Sex Reprod Healthc. 2017;13:83–90.
Bookari K, Yeatman H, Williamson M. Falling short of dietary guidelines – what do Australian pregnant women really know? A cross sectional study. Women Birth. 2017;30:9–17.
Rockliffe L, Peters S, Heazell AEP, Smith DM. Understanding pregnancy as a teachable moment for behaviour change: a comparison of the COM-B and teachable moments models. Heal Psychol Behav Med. 2022;10:41–59.
O’Brien OA, Lindsay KL, McCarthy M, McGloin AF, Kennelly M, Scully HA, et al. Influences on the food choices and physical activity behaviours of overweight and obese pregnant women: a qualitative study. Midwifery. 2017;47:28–35.
Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Educ Couns. 2011;83:203–9.
Cantor AG, Jungbauer RM, McDonagh M, Blazina I, Marshall NE, Weeks C, et al. Counseling and behavioral interventions for healthy weight and weight gain in pregnancy: evidence report and systematic review for the US Preventive Services Task Force. JAMA - J Am Med Assoc. 2021;325:2094–109.
Murphy M, McHugh S, O’Keeffe LM, Greene RA, Corcoran P, Kearney PM. Preventive health counselling during antenatal care using the pregnancy risk assessment monitoring system (PRAMS) in Ireland. BMC Pregnancy Childbirth. 2020;20:98.
Esfandiari M, Faramarzi M, Nasiri-Amiri F, Parsian H, Chehrazi M, Pasha H, et al. Effect of supportive counseling on pregnancy-specific stress, general stress, and prenatal health behaviors: a multicenter randomized controlled trial. Patient Educ Couns. 2020;103:2297–304.
Acknowledgements
Authors thank to Michael J. McConnell for his valuable support.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by “Convocatoria IMIENS de ayudas para la realización de Proyectos de Iniciación a la Investigación 2022” awarded to E.G-R and M.R-M. E.G-R. is supported by the Sara Borrell Program (CD18CIII/00007), Instituto de Salud Carlos III, Ministerio de Ciencia, Innovación y Universidades and the Generalitat Valenciana plan GenT grant No. CIDEIG/2022/028. C.M-M. is supported by the PFIS Program (FI22CIII/0029), Instituto de Salud Carlos III, Ministerio de Ciencia, Innovación y Universidades. The accreditation to the IATA-CSIC as center of Excellence Severo Ochoa CEX2021-001189-S funded by MCIU/https://doi.org/10.13039/501100011033 is also fully acknowledged.
Author information
Authors and Affiliations
Contributions
MRF: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Supervision, Funding acquisition. CMM: Investigation, Original Draft, Writing. KK: Investigation, Original Draft, Writing. MEO: Investigation, Original Draft, Writing. NI: Investigation, Original Draft, Writing. PPR: Investigation, Original Draft, Writing. EGR: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
None.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rodríguez-Muñoz, M.F., Martín-Martín, C., Kovacheva, K. et al. Hygiene-based measures for the prevention of cytomegalovirus infection in pregnant women: a systematic review. BMC Pregnancy Childbirth 24, 172 (2024). https://doi.org/10.1186/s12884-024-06367-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06367-5